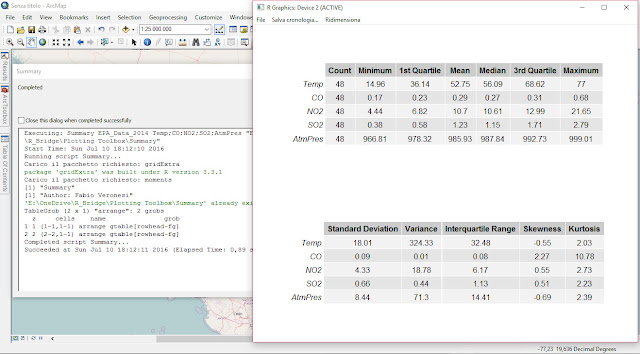
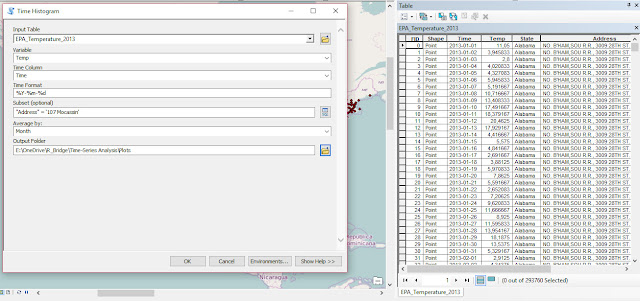
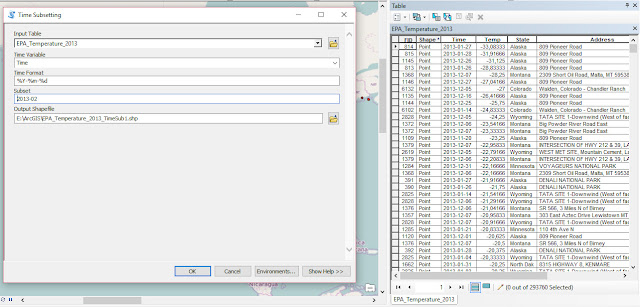
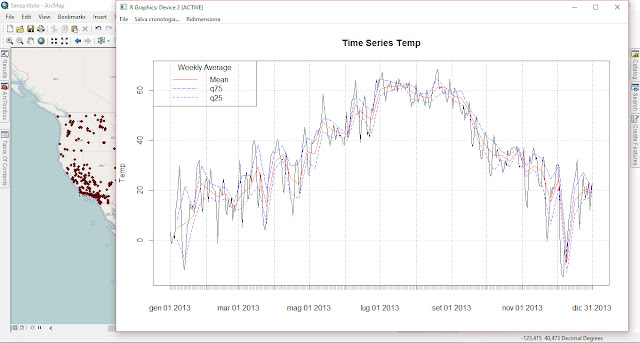
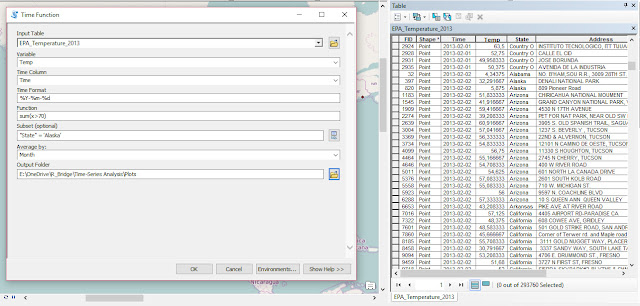
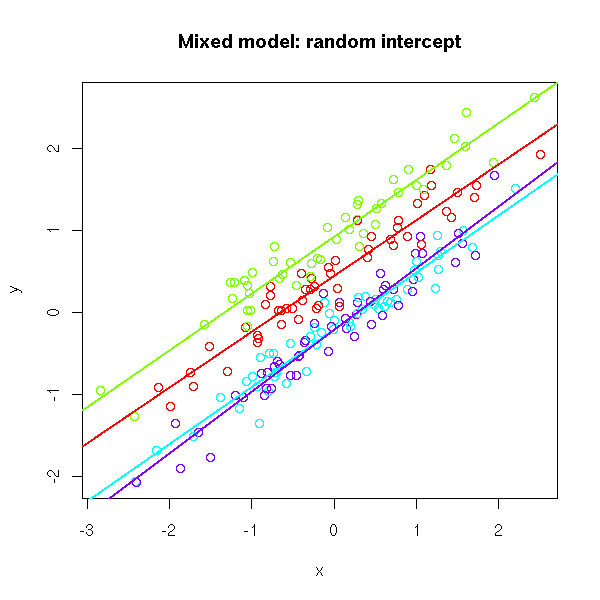
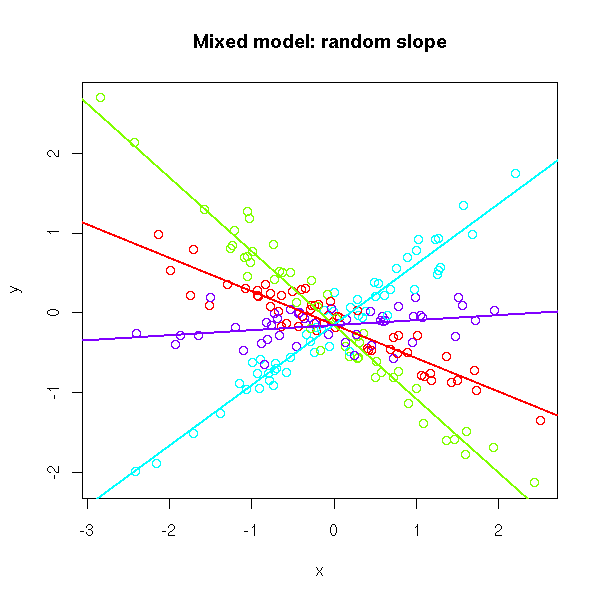
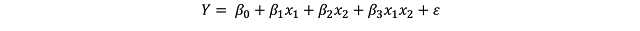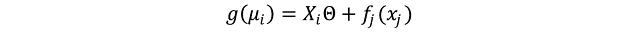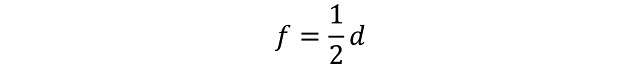As part of my new role as Lecturer in Agri-data analysis at Harper Adams University, I found myself applying a lot of techniques based on linear modelling. Another thing I noticed is that there is a lot of confusion among researchers in regards to what technique should be used in each instance and how to interpret the model. For this reason I started reading material from books and on-line to try and create a sort of reference tutorial that researchers can use. This post is the result of my work so far and I will keep updating it with new information.
Please feel free to comment, provide feedback and constructive criticism!!
Theoretical Background - Linear Model and ANOVA
Linear Model
The classic linear model forms the basis for ANOVA (with categorical treatments) and ANCOVA (which deals with continuous explanatory variables). Its basic equation is the following:
where β_0 is the intercept (i.e. the value of the line at zero), β_1 is the slope for the variable x, which indicates the changes in y as a function of changes in x. For example if the slope is +0.5, we can say that for each unit increment in x, y increases of 0.5. Please note that the slope can also be negative. The last element of the equation is the random error term, which we assume normally distributed with mean zero and constant variance.
This equation can be expanded to accommodate more that one explanatory variable x:
In this case the interpretation is a bit more complex because for example the coefficient β_2 provides the slope for the explanatory variable x_2. This means that for a unit variation of x_2 the target variable y changes by the value of β_2, if the other explanatory variables are kept constant.
In case our model includes interactions, the linear equation would be changed as follows:
notice the interaction term between x_1 and x_2. In this case the interpretation becomes extremely difficult just by looking at the model.
In fact, if we rewrite the equation focusing for example on x_1:
we can see that its slope become affected by the value of x_2 (Yan & Su, 2009), for this reason the only way we can actually determine how x_1 changes Y, when the other terms are kept constant, is to use the equation with new values of x_1.
This linear model can be applied to continuous target variables, in this case we would talk about an ANCOVA for exploratory analysis, or a linear regression if the objective was to create a predictive model.
ANOVA
The Analysis of variance is based on the linear model presented above, the only difference is that its reference point is the mean of the dataset. When we described the equations above we said that to interpret the results of the linear model we would look at the slope term; this indicates the rate of changes in Y if we change one variable and keep the rest constant. The ANOVA calculates the effects of each treatment based on the grand mean, which is the mean of the variable of interest.
In mathematical terms ANOVA solves the following equation (Williams, 2004):
where y is the effect on group j of treatment τ_1, while μ is the grand mean (i.e. the mean of the whole dataset). From this equation is clear that the effects calculated by the ANOVA are not referred to unit changes in the explanatory variables, but are all related to changes on the grand mean.
Examples of ANOVA and ANCOVA in R
For this example we are going to use one of the datasets available in the package agridatavailable in CRAN:
install.packages("agridat")
We also need to include other packages for the examples below. If some of these are not installed in your system please use again the function install.packages (replacing the name within quotation marks according to your needs) to install them.
library(agridat)
library(ggplot2)
library(plotrix)
library(moments)
library(car)
library(fitdistrplus)
library(nlme)
library(multcomp)
library(epade)
library(lme4)
Now we can load the dataset lasrosas.corn, which has more that 3400 observations of corn yield in a field in Argentina, plus several explanatory variables both factorial (or categorical) and continuous.
> dat = lasrosas.corn
> str(dat)
'data.frame': 3443 obs. of 9 variables:
$ year : int 1999 1999 1999 1999 1999 1999 1999 1999 1999 1999 ...
$ lat : num -33.1 -33.1 -33.1 -33.1 -33.1 ...
$ long : num -63.8 -63.8 -63.8 -63.8 -63.8 ...
$ yield: num 72.1 73.8 77.2 76.3 75.5 ...
$ nitro: num 132 132 132 132 132 ...
$ topo : Factor w/ 4 levels "E","HT","LO",..: 4 4 4 4 4 4 4 4 4 4 ...
$ bv : num 163 170 168 177 171 ...
$ rep : Factor w/ 3 levels "R1","R2","R3": 1 1 1 1 1 1 1 1 1 1 ...
$ nf : Factor w/ 6 levels "N0","N1","N2",..: 6 6 6 6 6 6 6 6 6 6 ...
Important for the purpose of this tutorial is the target variable yield, which is what we are trying to model, and the explanatory variables: topo (topographic factor), bv (brightness value, which is a proxy for low organic matter content) and nf (factorial nitrogen levels). In addition we have rep, which is the blocking factor.
Checking Assumptions
Since we are planning to use an ANOVA we first need to check that our data fits with its assumptions. ANOVA is based on three assumptions:
- Independence, in terms of independence of the error term
- Normality of the response variable (y)
- Normality of the error term (i.e. residuals).
- Equality of variances between groups
- Balance design (i.e. all groups have the same number of samples)
NOTE:
Normality of the response variable is a contested point and not all authors agree with this. In my reading I found some author explicitly talk about normality of the response variable, while others only talk about normality of the errors. In the R Book the author states as assumption only normality of error, but says that the ANOVA can be applied to random variables, which in a way should imply normality of the response.
Let’s see how we can test for them in R. Clearly we are talking about environmental data so the assumption of independence is not met, because data are autocorrelated with distance. Theoretically speaking, for spatial data ANOVA cannot be employed and more robust methods should be employed (e.g. REML); however, over the years it has been widely used for analysis of environmental data and it is accepted by the community. That does not mean that it is the correct method though, and later on in this tutorial we will see the function to perform linear modelling with REML.
The third assumption is the one that is most easy to assess using the function tapply:
> tapply(dat$yield, INDEX=dat$nf, FUN=var)
N0 N1 N2 N3 N4 N5
438.5448 368.8136 372.8698 369.6582 366.5705 405.5653
In this case we used tapply to calculate the variance of yield for each subgroup (i.e. level of nitrogen). There is some variation between groups but in my opinion it is not substantial. Now we can shift our focus on normality. There are tests to check for normality, but again the ANOVA is flexible (particularly where our dataset is big) and can still produce correct results even when its assumptions are violated up to a certain degree. For this reason, it is good practice to check normality with descriptive analysis alone, without any statistical test. For example, we could start by plotting the histogram of yield:
hist(dat$yield, main="Histogram of Yield", xlab="Yield (quintals/ha)")
qqnorm(dat$yield, main="QQplot of Yield")
qqline(dat$yield)
For normally distributed data the points should all be on the line. This is clearly not the case but again the deviation is not substantial. The final element we can calculate is the skewness of the distribution, with the function skewnessin the package moments:
> skewness(dat$yield)
[1] 0.3875977
According to Webster and Oliver (2007) is the skewness is below 0.5, we can consider the deviation from normality not big enough to transform the data. Moreover, according to Witte and Witte (2009) if we have more than 10 samples per group we should not worry too much about violating the assumption of normality or equality of variances.
To see how many samples we have for each level of nitrogen we can use once again the function tapply:
> tapply(dat$yield, INDEX=dat$nf, FUN=length)
N0 N1 N2 N3 N4 N5
573 577 571 575 572 575
As you can see we have definitely more than 10 samples per group, but our design is not balanced (i.e. some groups have more samples). This implies that the normal ANOVA cannot be used, this is because the standard way of calculating the sum of squares is not appropriate for unbalanced designs (look here for more info:
http://goanna.cs.rmit.edu.au/~fscholer/anova.php).
The same function tapply can be used to check the assumption of equality of variances. We just need to replace the function length with the function var for the option FUN.
In summary, even though from the descriptive analysis it appears that our data are close to being normal and have equal variance, our design is unbalanced, therefore the normal way of doing ANOVA cannot be used. In other words we cannot function aovfor this dataset. However, since this is a tutorial we are still going to start by applying the normal ANOVA with aov.
ANOVA with aov
The first thing we need to do is think about the hypothesis we would like to test. For example, we could be interested in looking at nitrogen levels and their impact on yield. Let’s start with some plotting to better understand our data:
means.nf = tapply(dat$yield, INDEX=dat$nf, FUN=mean)
StdErr.nf = tapply(dat$yield, INDEX=dat$nf, FUN= std.error)
BP = barplot(means.nf, ylim=c(0,max(means.nf)+10))
segments(BP, means.nf - (2*StdErr.nf), BP,
means.nf + (2*StdErr.nf), lwd = 1.5)
arrows(BP, means.nf - (2*StdErr.nf), BP,
means.nf + (2*StdErr.nf), lwd = 1.5, angle = 90,
code = 3, length = 0.05)
This code first uses the function tapply to compute mean and standard error of the mean for yield in each nitrogen group. Then it plots the means as bars and creates error bars using the standard error (please remember that with a normal distribution ± twice the standard error provides a 95% confidence interval around the mean value). The result is the following image:
![]()
By plotting our data we can start figuring out what is the interaction between nitrogen levels and yield. In particular, there is an increase in yield with higher level of nitrogen. However, some of the error bars are overlapping, and this may suggest that their values are not significantly different. For example, by looking at this plot N0 and N1 have error bars very close to overlap, but probably not overlapping, so it may be that N1 provides a significant different from N0. The rest are all probably significantly different from N0. For the rest their interval overlap most of the times, so their differences would probably not be significant.
We could formulate the hypothesis that nitrogen significantly affects yield and that the mean of each subgroup are significantly different. Now we just need to test this hypothesis with a one-way ANOVA:
mod1 = aov(yield ~ nf, data=dat)
The code above uses the function aov to perform an ANOVA; we can specify to perform a one-way ANOVA simply by including only one factorial term after the tilde (~) sign. We can plot the ANOVA table with the function summary:
> summary(mod1)
Df Sum Sq Mean Sq F value Pr(>F)
nf 5 23987 4797 12.4 6.08e-12 ***
Residuals 3437 1330110 387
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
It is clear from this output that nitrogen significantly affects yield, so we tested our first hypothesis. To test the significance for individual levels of nitrogen we can use the Tukey’s test:
> TukeyHSD(mod1, conf.level=0.95)
Tukey multiple comparisons of means
95% family-wise confidence level
Fit: aov(formula = yield ~ nf, data = dat)
$nf
diff lwr upr p adj
N1-N0 3.6434635 0.3353282 6.951599 0.0210713
N2-N0 4.6774357 1.3606516 7.994220 0.0008383
N3-N0 5.3629638 2.0519632 8.673964 0.0000588
N4-N0 7.5901274 4.2747959 10.905459 0.0000000
N5-N0 7.8588595 4.5478589 11.169860 0.0000000
N2-N1 1.0339723 -2.2770686 4.345013 0.9489077
N3-N1 1.7195004 -1.5857469 5.024748 0.6750283
N4-N1 3.9466640 0.6370782 7.256250 0.0089057
N5-N1 4.2153960 0.9101487 7.520643 0.0038074
N3-N2 0.6855281 -2.6283756 3.999432 0.9917341
N4-N2 2.9126917 -0.4055391 6.230923 0.1234409
N5-N2 3.1814238 -0.1324799 6.495327 0.0683500
N4-N3 2.2271636 -1.0852863 5.539614 0.3916824
N5-N3 2.4958957 -0.8122196 5.804011 0.2613027
N5-N4 0.2687320 -3.0437179 3.581182 0.9999099
There are significant differences between the control and the rest of the levels of nitrogen, plus other differences between N4 and N5 compared to N1, but nothing else. If you look back at the bar chart we produced before, and look carefully at the overlaps between error bars, you will see that for example N1, N2, and N3 have overlapping error bars, thus they are not significantly different. On the contrary, N1 has no overlaps with either N4 and N5 , which is what we demonstrated in the ANOVA.
The function model.tables provides a quick way to print the table of effects and the table of means:
> model.tables(mod1, type="effects")
Tables of effects
nf
N0 N1 N2 N3 N4 N5
-4.855 -1.212 -0.178 0.5075 2.735 3.003
rep 573.000 577.000 571.000 575.0000 572.000 575.000
These values are all referred to the gran mean, which we can simply calculate with the function mean(dat$yield) and it is equal to 69.83. This means that the mean for N0 would be 69.83-4.855 = 64.97. we can verify that with another call to the function model.tables, this time with the option type=”means”:
> model.tables(mod1, type="means")
Tables of means
Grand mean
69.82831
nf
N0 N1 N2 N3 N4 N5
64.97 68.62 69.65 70.34 72.56 72.83
rep 573.00 577.00 571.00 575.00 572.00 575.00
Linear Model with 1 factor
The same results can be obtain by fitting a linear model with the function lm, only their interpretation would be different. The assumption for fitting a linear models are again independence (which is always violated with environmental data), and normality.
Let’s look at the code:
mod2 = lm(yield ~ nf, data=dat)
This line fits the same model but with the standard linear equation. This become clearer by looking at the summary table:
> summary(mod2)
Call:
lm(formula = yield ~ nf, data = dat)
Residuals:
Min 1Q Median 3Q Max
-52.313 -15.344 -3.126 13.563 45.337
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 64.9729 0.8218 79.060 < 2e-16 ***
nfN1 3.6435 1.1602 3.140 0.0017 **
nfN2 4.6774 1.1632 4.021 5.92e-05 ***
nfN3 5.3630 1.1612 4.618 4.01e-06 ***
nfN4 7.5901 1.1627 6.528 7.65e-11 ***
nfN5 7.8589 1.1612 6.768 1.53e-11 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 19.67 on 3437 degrees of freedom
Multiple R-squared: 0.01771, Adjusted R-squared: 0.01629
F-statistic: 12.4 on 5 and 3437 DF, p-value: 6.075e-12
There are several information in this table that we should clarify. First of all it already provides with some descriptive measures for the residuals, from which we can see that their distribution is relatively normal (first and last quartiles have similar but opposite values and the same is true for minimum and maximum). As you remember when we talked about assumptions, one was that the error term is normal. This first part of the output allows us to check whether we are meeting this assumption.
Other important information we should look at are the R-squared and Adjusted R-squared (please look at the end of the page to know more about these two values). In essence, R-squared tells us how much variance in the data can be explained by the model, in this case not much. However, this is an exploratory rather than a predictive model, so we know that there may be other factors that affect the variability of yield, but we are not interested in them. We are only interested in understanding in the impact of the level of nitrogen. Another important information is the F-statistics at the end, with the p-value (which is very low). The F-statistic is the ration between the variability between groups (meaning between different level of N) and within groups (meaning the variability with samples with the same value of N). This ratio and the related p-value tell us that our model is significant (because the variability that we obtain increasing N is higher that the normal variability we expect from random variation), which means that nitrogen has an effect on yield.
Then we have the table of the coefficients, with the intercept and all the slopes, plus their standard errors. These can be used to build confidence intervals for the coefficients, which are used to assess the uncertainty around their estimation. We can actually compute the confidence intervals with the function confint (the option level is used to specify for example that we are looking at the 95% confidence interval):
> confint(mod2, level = 0.95)
2.5 % 97.5 %
(Intercept) 63.361592 66.584202
nfN1 1.368687 5.918240
nfN2 2.396712 6.958160
nfN3 3.086217 7.639711
nfN4 5.310402 9.869853
nfN5 5.582112 10.135607
Another important element about coefficients is that there measuring unit is the same as the dependent variable, because it provides an estimate of the effect of a predictor to the dependent variable, i.e. yield.
As you can see the level N0 is not shown in the list; this is called the reference level, which means that all the other are referenced back to it. In other words, the value of the intercept is the mean of nitrogen level 0 (in fact is the same we calculated above 64.97). To calculate the means for the other groups we need to sum the value of the reference level with the slopes. For example N1 is 64.97 + 3.64 = 68.61 (the same calculated from the ANOVA). The p-value and the significance are again in relation to the reference level, meaning for example that N1 is significantly different from N0 (reference level) and the p-value is 0.0017. This is similar to the Tukey’s test we performed above, but it is only valid in relation to N0. As you can see the p-value is computed from the t-statistic, this is because R computes t-test comparing all the factors to the reference level.
We need to change the reference level, and fit another model, to get the same information for other nitrogen levels:
dat$nf = relevel(dat$nf, ref="N1")
mod3 = lm(yield ~ nf, data=dat)
summary(mod3)
Now the reference level is N1, so all the results will tell us the effects of nitrogen in relation to N1.
> summary(mod3)
Call:
lm(formula = yield ~ nf, data = dat)
Residuals:
Min 1Q Median 3Q Max
-52.313 -15.344 -3.126 13.563 45.337
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 68.616 0.819 83.784 < 2e-16 ***
nfN0 -3.643 1.160 -3.140 0.001702 **
nfN2 1.034 1.161 0.890 0.373308
nfN3 1.720 1.159 1.483 0.138073
nfN4 3.947 1.161 3.400 0.000681 ***
nfN5 4.215 1.159 3.636 0.000280 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 19.67 on 3437 degrees of freedom
Multiple R-squared: 0.01771, Adjusted R-squared: 0.01629
F-statistic: 12.4 on 5 and 3437 DF, p-value: 6.075e-12
For example, we can see that N0 has a lower value compared to N1, and that only N0, N4 and N5 are significantly different from N1, which is what we saw from the bar chart and what we found from the Tukey’s test.
Interpreting the output of the function aov is much easier compare to lm. However, in many cases we can only use the function lm (for example in an ANCOVA where alongside categorical we have continuous explanatory variables) so it is important that we learn how to interpret its summary table.
We can obtain the ANOVA table with the function anova:
> anova(mod2)
Analysis of Variance Table
Response: yield
Df Sum Sq Mean Sq F value Pr(>F)
nf 5 23987 4797.4 12.396 6.075e-12 ***
Residuals 3437 1330110 387.0
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
This uses the type I sum of squares (more info at: http://www.utstat.utoronto.ca/reid/sta442f/2009/typeSS.pdf), which is the standard way and it is not indicated for unbalanced designs. The function Anova in the package car has the option to select which type of sum of squares to calculate and we can specify type=c(“III”)to correct for the unbalanced design:
> Anova(mod2, type=c("III"))
Anova Table (Type III tests)
Response: yield
Sum Sq Df F value Pr(>F)
(Intercept) 2418907 1 6250.447 < 2.2e-16 ***
nf 23987 5 12.396 6.075e-12 ***
Residuals 1330110 3437
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
In this example the two results are the same, probably the large sample size helps in this respect. However, for smaller samples this distinction may become important. For this reason, if your design is unbalanced please remember not to use the function aov, but always lmand Anova with the option for type III sum of squares.
Two-way ANOVA
So far we have looked on the effect of nitrogen on yield. However, in the dataset we also have a factorial variable named topo, which stands for topographic factor and has 4 levels: W = West slope, HT = Hilltop, E = East slope, LO = Low East. We already formulated an hypothesis about nitrogen, so now we need to formulate an hypothesis about topo as well. Once again we can do that by using the function tapply and a simple bar charts with error bars. Look at the code below:
means.topo = tapply(dat$yield, INDEX=dat$topo, FUN=mean)
StdErr.topo = tapply(dat$yield, INDEX=dat$topo, FUN= std.error)
BP = barplot(means.topo, ylim=c(0,max(means.topo)+10))
segments(BP, means.topo - (2*StdErr.topo), BP,
means.topo + (2*StdErr.topo), lwd = 1.5)
arrows(BP, means.topo - (2*StdErr.topo), BP,
means.topo + (2*StdErr.topo), lwd = 1.5, angle = 90,
code = 3, length = 0.05)
Here we are using the same exact approach we used before to formulate an hypothesis about nitrogen. We first calculate mean and standard error of yield for each level of topo, and then plot a bar chart with error bars.
The result is the plot below:
From this plot it is clear that the topographic factor has an effect on yield. In particular, hilltop areas have low yield while the low east corner of the field has high yield. From the error bars we can say with a good level of confidence that probably all the differences will be significant, at least up to an alpha of 95% (significant level, meaning a p-value of 0.05).
We can test this hypothesis with a two way ANOVA, by simply adding the term topo to the equation:
mod1b = aov(yield ~ nf + topo, data=dat)
summary(mod1b)
Df Sum Sq Mean Sq F value Pr(>F)
nf 5 23987 4797 23.21 <2e-16 ***
topo 3 620389 206796 1000.59 <2e-16 ***
Residuals 3434 709721 207
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
From the summary table it is clear that both factors have a significant effect on yield, but just by looking at this it is very difficult to identify clearly which levels are the significant ones. Top do that we need the Tukey’s test:
TukeyHSD(mod1b, conf.level=0.95, which=c("topo"))
Tukey multiple comparisons of means
95% family-wise confidence level
Fit: aov(formula = yield ~ nf + topo, data = dat)
$topo
diff lwr upr p adj
HT-LO -36.240955 -38.052618 -34.429291 0
W-LO -18.168544 -19.857294 -16.479794 0
E-LO -6.206619 -8.054095 -4.359143 0
W-HT 18.072411 16.326440 19.818381 0
E-HT 30.034335 28.134414 31.934257 0
E-W 11.961925 10.178822 13.745028 0
The zero p-values indicate a large significance for each combination, as it was clear from the plot. With the function model.table you can easily obtain a table of means or effects, if you are interested.
Two-Way ANOVA with Interactions
One step further we can take to get more insights into our data is add an interaction between nitrogen and topo, and see if this can further narrow down the main sources of yield variation. Once again we need to start our analysis by formulating an hypothesis. Since we are talking about an interaction we are now concern in finding a way to plot yield responses for varying nitrogen level and topographic position, so we need a 3d bar chart. We can do that with the function bar3d.ade from the package epade, so please install this package and load it.
Then please look at the following R code:
dat$topo = factor(dat$topo,levels(dat$topo)[c(2,4,1,3)])
means.INT = tapply(dat$yield, INDEX=list(dat$nf, dat$topo), FUN=mean)
bar3d.ade(means.INT, col="grey")
The first line is only used to reorder the levels in the factorial variable topo. This is because from the previous plot we clearly saw that HT is the one with the lowest yield, followed by W, E and LO. We are doing this only to make the 3d bar chart more readable.
The next line applies once again the function tapply, this time to calculate the mean of yield for subgroups divided by nitrogen and topographic factors. The result is a matrix that looks like this:
> means.INT
LO HT W E
N0 81.03027 41.50652 62.08192 75.13902
N1 83.06276 48.33630 65.74627 78.12808
N2 85.06879 48.79830 66.70848 78.92632
N3 85.23255 50.18398 66.16531 78.99210
N4 87.14400 52.12039 70.10682 80.39213
N5 87.94122 51.03138 69.65933 80.55078
This can be used directly within the function bar3d.ade to create the 3d bar chart below:
![]()
From this plot we can see two things very clearly: the first is that there is an increase in yield from HT to LO in the topographic factor, the second is that we have again and increase from N0 to N1 in the nitrogen levels. These were all expected since we already noticed them before. What we do not see in these plot is any particular influence from the interaction between topography and nitrogen. For example, if you look at HT, you have an increase in yield from N0 to N5 (expected) and overall the yield is lower than the other bars (again expected). If there was an interaction we would expect this general pattern to change, for example with relatively high yield on the hilltop at high nitrogen level, or very low yield in the low east side with N0. This does not happen and all the bars follow an expected pattern, so we can hypothesise that the interaction will not be significant.
We can further explore a possible interaction between nf and topo by creating an interaction plot:
with(dat, interaction.plot(topo, nf, yield))
This line applies the function interaction plot within the call to the function with, which indicates to R that the names are referred to the dataset named dat. The result is the following image:
Again, all the lines increase with changes in topography, but there no additional effect provided by changes in nf. In fact, the lines never cross, or just cross slightly: this is a good indication of lack of interaction.
To formally test our hypothesis of lack of interaction, we need to run another ANOVA with an interaction term:
mod1c = aov(yield ~ nf * topo, data=dat)
This formula test for both main effects and their interaction. To see the significance we can use the summary table:
> summary(mod1c)
Df Sum Sq Mean Sq F value Pr(>F)
nf 5 23987 4797 23.176 <2e-16 ***
topo 3 620389 206796 999.025 <2e-16 ***
nf:topo 15 1993 133 0.642 0.842
Residuals 3419 707727 207
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
From this we can conclude that our hypothesis was correct and that the interaction has no effect on yield.
We can have a even better ides of the interaction effect by using some functions in the package phia:
library(phia)
plot(interactionMeans(mod1c))
This function plots the effects of the interactions in a 2 by 2 plot, including the standard error of the coefficients, so that we can readily see which overlap:
We already knew from the 3d plot that there is a general increase between N0 and N5 that mainly drives the changes we see in the data. However, from the top-right plot we can see that topo plays a little role between N0 and the other (in fact the black line only slightly overlap with the other), but it has no effect on N1 to N5.
We can look at the numerical break-out of what we see in the plot with another function:
> testInteractions(mod1c)
F Test:
P-value adjustment method: holm
Value Df Sum of Sq F Pr(>F)
N0-N1 : HT-W -3.1654 1 377 1.8230 1
N0-N2 : HT-W -2.6652 1 267 1.2879 1
N0-N3 : HT-W -4.5941 1 784 3.7880 1
N0-N4 : HT-W -2.5890 1 250 1.2072 1
N0-N5 : HT-W -1.9475 1 140 0.6767 1
N1-N2 : HT-W 0.5002 1 9 0.0458 1
N1-N3 : HT-W -1.4286 1 76 0.3694 1
N1-N4 : HT-W 0.5765 1 12 0.0604 1
N1-N5 : HT-W 1.2180 1 55 0.2669 1
N2-N3 : HT-W -1.9289 1 139 0.6711 1
N2-N4 : HT-W 0.0762 1 0 0.0011 1
N2-N5 : HT-W 0.7178 1 19 0.0924 1
N3-N4 : HT-W 2.0051 1 149 0.7204 1
The table is very long so only the first lines are included. However, from this it is clear that the interaction has no effect (p-value of 1), but if it was this function can give us numerous details about the specific effects.
Now we could try to compare the two models to see if they are different in the amount of variability they can explain in the data. This can be done with the function anova, and performing an F test:
> anova(mod1b, mod1c, test="F")
Analysis of Variance Table
Model 1: yield ~ nf + topo
Model 2: yield ~ nf * topo
Res.Df RSS Df Sum of Sq F Pr(>F)
1 3434 709721
2 3419 707727 15 1993.2 0.6419 0.8421
As we can see from this output, the p-value is not significant. This means that the two model are no different in their explanatory power. This further support the fact that including an interaction does not change the accuracy of the model, and probably decreases it. We could test this last statement for example by looking at the AIC for both models, we will see how to do that later on in the tutorial.
Please remember that the method we just used can be employed to compare probably all the models we are going to fit in this tutorial, so it is a very powerful method!
ANCOVA with lm
The Analysis of covariance (ANCOVA) fits a new model where the effects of the treatments (or factorial variables) is corrected for the effect of continuous covariates, for which we can also see the effects on yield.
The code is very similar to what we saw before, and again we can perform an ANCOVA with the lm function; the only difference is that here we are including an additional continuous explanatory variable in the model:
> mod3 = lm(yield ~ nf + bv, data=dat)
> summary(mod3)
Call:
lm(formula = yield ~ nf + bv, data = dat)
Residuals:
Min 1Q Median 3Q Max
-78.345 -10.847 -3.314 10.739 56.835
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 271.55084 4.99308 54.385 < 2e-16 ***
nfN0 -3.52312 0.95075 -3.706 0.000214 ***
nfN2 1.54761 0.95167 1.626 0.103996
nfN3 2.08006 0.94996 2.190 0.028619 *
nfN4 3.82330 0.95117 4.020 5.96e-05 ***
nfN5 4.47993 0.94994 4.716 2.50e-06 ***
bv -1.16458 0.02839 -41.015 < 2e-16 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 16.12 on 3436 degrees of freedom
Multiple R-squared: 0.3406, Adjusted R-squared: 0.3394
F-statistic: 295.8 on 6 and 3436 DF, p-value: < 2.2e-16
By printing the summary table we can already see some differences compared to the model we only nitrogen as explanatory variable. The first is related to the Adjusted R-squared (which is simply the R-squared corrected for the number of predictors so that it is less affected by overfitting), which in this case is around 0.3. If we look back at the summary table of the model with only nitrogen, the R-squared was only 0.01. This means that by adding the continuous variable bv we are able to massively increase the explanatory power of the model; in fact, this new model is capable of explaining 33% of the variation in yield. Moreover, we can also see that other terms become significant, for example N3. This is because the inclusion of bv changes the entire model and its interpretation becomes less obvious compared to the simple bar chart we plotted at the beginning.
The interpretation of the ANCOVA model is more complex that the one for the one-way ANOVA. In fact, the intercept value has changed and it is not the mean of the reference level N1. This is because the model now changes based on the covariate bv. The slope can be used to assess the relative impact of each term; for example, N0 has a negative impact on yield in relation to its reference level. Therefore, shifting from a nitrogen level N1 to N0 decreases the yield by -3.52, if bv is kept constant.
Take a look at the following code:
> test.set = data.frame(nf="N1", bv=150)
> predict(mod3, test.set)
1 2
96.86350 38.63438
>
> test.set = data.frame(nf="N0", bv=150)
> predict(mod3, test.set)
1
93.34037
Here we are using the model (mod3) to estimate new values of yield based on set parameters. In the first example we set nf to N1 (reference level) and bv constant at 150. With the function predictwe can see estimate these new values using mod3. For N1 and bv equal to 150 the yield is 96.86. In the second example we did the same but for nitrogen level N0. The result is a yield equal to 93.34, that is a difference of exactly 3.52, which is the slope of the model.
For computing the ANOVA table, we can again use either the function
anova (if the design is balanced) or
Anova with type III (for unbalanced designs).
Let's now look at some diagnostic plots we can use to test whether our model meets all the assumptions for linear models. We can use the default plot function in R to do so:
par(mfrow=c(2,2))
plot(mod3)
Here I first used the function par, to divide the plotting window into 2 rows and two columns so that we can plot all four diagnostic plots into the same window. The result is the following:
The top left plot is represents the residuals against the fitted values (or the estimates from the model). One of our assumptions was that the error term had mean equal to zero and constant variance. This means that we should see the residuals equally spread around zero. We should see a more or less horizontal line with intercept on the zero. In fact, we actually see that for low values of yield (x axis) we have a sort of equal spread around zero, but this changes with the increase in yield; this is clearly a violation of the assumption. The second plot on the top represents the QQplot of the residuals, which again should be on the middle line because another assumption is that the error should be normal. Again we have some values that do not fit with normality. A good thing is that R prints the ID of these values so that we can evaluate whether we think they are outliers or we have another explanation for the violations of the assumptions.
In the second row on the left we have a plot that again is used to check whether we meet the assumption of constant variance. We should again see a more or less horizontal line, but again we have an increase in variance, which violates the assumption. Finally, we have the residuals vs. leverage plot and the Cook's Distance. Leverage represents the influence of each point on the model; again we see that some points have larger influence on the model. This should not be the case, we should see again a more or less equal spread of point. Another information we can have from this plot is whether the extreme observations may be outliers. If our extreme points would lie outside the Cook's Distance zone we would suspect them to be outliers. However, in this case the zone is not even plotted because it is outside the plotting area, so we probably do not have outliers.
For more info about diagnostic plots please take a look here:
http://data.library.virginia.edu/diagnostic-plots/
http://www.statmethods.net/stats/rdiagnostics.html
We can further investigate as to why our model does not meet all assumptions by looking at the residuals vs. fitted values and try to color the points based for example on topo. We can do that easily with ggplot2:
qplot(fitted(mod3), residuals(mod3), col=dat$topo, geom="point", xlab="Fitted Values", ylab="Residuals")
This creates the following image:
From this image it is clear that all the points that look as possible outliers come from a specific topographic category. This may mean different things depending on the data. In this case I think it only means that we should include topo in our model. However, for other data it may mean that we should exclude certain categories, but the point I want to make is that it is always important not to look only at the summary table but try to explore the model a bit more in details to draw more meaningful conclusions.
Two-factors and one continuous explanatory variable
Let’s look now at another example with a slightly more complex model where we include two factorial and one continuous variable. We also include in the model the variable topo. We can check these with the function levels:
> levels(dat$topo)
[1] "E""HT""LO""W"
Please notice that E is the first and therefore is the reference level for this factor. Now let’s fit the model and look at the summary table:
> mod4 = lm(yield ~ nf + bv + topo, data=dat)
>
> summary(mod4)
Call:
lm(formula = yield ~ nf + bv + topo, data = dat)
Residuals:
Min 1Q Median 3Q Max
-46.394 -10.927 -2.211 10.364 47.338
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 171.20921 5.28842 32.374 < 2e-16 ***
nfN0 -3.73225 0.81124 -4.601 4.36e-06 ***
nfN2 1.29704 0.81203 1.597 0.1103
nfN3 1.56499 0.81076 1.930 0.0537 .
nfN4 3.71277 0.81161 4.575 4.94e-06 ***
nfN5 3.88382 0.81091 4.789 1.74e-06 ***
bv -0.54206 0.03038 -17.845 < 2e-16 ***
topoHT -24.11882 0.78112 -30.877 < 2e-16 ***
topoLO 3.13643 0.70924 4.422 1.01e-05 ***
topoW -10.77758 0.66708 -16.156 < 2e-16 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 13.75 on 3433 degrees of freedom
Multiple R-squared: 0.5204, Adjusted R-squared: 0.5191
F-statistic: 413.8 on 9 and 3433 DF, p-value: < 2.2e-16
The adjusted R-squared increases again and now we are able to explain around 52% of the variation in yield.
The interpretation is similar to what we said before, the only difference is that here both factors have a reference level. So for example, the effect of topoHT is related to the reference level, which is the one not shown E. So if we change the topographic position from E to HT, while keeping everything else in the model constant (meaning same value of bv and same nitrogen level), we obtain a decrease in yield of 24.12.
Another thing we can see from this table is that the p-value change, and for example N3 becomes less significant. This is probably because when we consider more variables the effect of N3 on yield is explained by other variables, maybe partly bv and partly topo.
One last thing we can check, and this is something we should check every time we perform an ANOVA or a linear model is the normality of the residuals. We already saw that the summary table provides us with some data about the residuals distribution (minimum, first quartile, median, third quartile and maximum) that gives us a good indication of normality, since the distribution is centred around 0. However, we can also use other tools to check this. For example a QQ plot:
RES = residuals(mod4)
qqnorm(RES)
qqline(RES)
The function residuals automatically extract the residuals from the model, which can then be used to create the following plot:
It looks approximately normal, but to have a further confirmation we can use again the function skewness, which returns a value below 0.5, so we can consider this a normal distribution.
ANCOVA with interactions
Let’s now add a further layer of complexity by adding an interaction term between bv and topo. Once again we need to formulate an hypothesis before proceeding to test it. Since we are interested in an interaction between a continuous variable (bv) and a factorial variable (topo) on yield, we could try to create scatterplots of yield versus bv, for the different levels in topo. We can easily do that with the package ggplot2:
qplot(yield, bv, data=dat, geom="point", xlab="Yield", ylab="bv") +
facet_wrap(~topo)+
geom_smooth(method = "lm", se = TRUE)
Explaining every bit of the three lines of code above would require some time and it is beyond the scope of this tutorial. In essence, these lines create a scatterplot yield versus bv for each subgroup of topo and then fit a linear regression line through the points. For more info about the use of ggplot2 please start by looking here:
http://www.statmethods.net/advgraphs/ggplot2.html
This create the plot below:
From this plot it is clear that the four lines have different slopes, so the interaction between bv and topo may well be significant and help us further increase the explanatory power of our model. We can test that by adding this interaction:
mod5 = lm(yield ~ nf + bv * topo, data=dat)
We can use the function Anova to check the significance:
> Anova(mod5, type=c("III"))
Anova Table (Type III tests)
Response: yield
Sum Sq Df F value Pr(>F)
(Intercept) 20607 1 115.225 < 2.2e-16 ***
nf 23032 5 25.758 < 2.2e-16 ***
bv 5887 1 32.920 1.044e-08 ***
topo 40610 3 75.691 < 2.2e-16 ***
bv:topo 36059 3 67.209 < 2.2e-16 ***
Residuals 613419 3430
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
As you can see this interaction is significant. To check the details we can look at the summary table:
> summary(mod5)
Call:
lm(formula = yield ~ nf + bv * topo, data = dat)
Residuals:
Min 1Q Median 3Q Max
-46.056 -10.328 -1.516 9.622 50.184
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 93.45783 8.70646 10.734 < 2e-16 ***
nfN1 3.96637 0.78898 5.027 5.23e-07 ***
nfN2 5.24313 0.79103 6.628 3.93e-11 ***
nfN3 5.46036 0.79001 6.912 5.68e-12 ***
nfN4 7.52685 0.79048 9.522 < 2e-16 ***
nfN5 7.73646 0.79003 9.793 < 2e-16 ***
bv -0.27108 0.04725 -5.738 1.04e-08 ***
topoW 88.11105 12.07428 7.297 3.63e-13 ***
topoE 236.12311 17.12941 13.785 < 2e-16 ***
topoLO -15.76280 17.27191 -0.913 0.3615
bv:topoW -0.41393 0.06726 -6.154 8.41e-10 ***
bv:topoE -1.21024 0.09761 -12.399 < 2e-16 ***
bv:topoLO 0.28445 0.10104 2.815 0.0049 **
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 13.37 on 3430 degrees of freedom
Multiple R-squared: 0.547, Adjusted R-squared: 0.5454
F-statistic: 345.1 on 12 and 3430 DF, p-value: < 2.2e-16
The R-squared is a bit higher, which means that we can explain more of the variability in yield by adding the interaction. For the interpretation, once again everything is related to the reference levels in the factors, even the interaction. So for example, bv:topoW tells us that the interaction between bv and topo changes the yield negatively if we change from HT to W, keeping everything else constant.
For information about individual changes we would need to use the model to estimate new data as we did for mod3.
GLS – For violations of independence
As we mentioned, there are certain assumptions we need to check before starting an analysis with linear models. Assumptions about normality and equality of variance can be relaxed, particularly if sample sizes are large enough. However, other assumptions for example balance in the design and independence tend to be stricter, and we need to be careful in violating them.
We can check for independence by looking at the correlation between predictors and coefficient directly in the summary table. We do that because we need to check the independence of the error (i.e. the residuals). If residuals are independent the correlation will be low.
> summary(mod5, cor=T)
[…]
Correlation of Coefficients:
(Intercept) nfN1 nfN2 nfN3 nfN4 nfN5 bv topoW topoE topoLO
nfN1 -0.05
nfN2 -0.04 0.50
nfN3 -0.05 0.50 0.50
nfN4 -0.05 0.50 0.50 0.50
nfN5 -0.05 0.50 0.50 0.50 0.50
bv -1.00 0.01 -0.01 0.01 0.00 0.00
topoW -0.72 0.00 0.00 0.00 0.00 0.00 0.72
topoE -0.51 0.01 0.02 0.03 0.01 0.02 0.51 0.37
topoLO -0.50 -0.02 -0.01 0.02 -0.01 0.02 0.50 0.36 0.26
bv:topoW 0.70 0.00 0.00 0.00 0.00 0.00 -0.70 -1.00 -0.36 -0.35
bv:topoE 0.48 -0.01 -0.02 -0.03 -0.01 -0.02 -0.48 -0.35 -1.00 -0.24
bv:topoLO 0.47 0.02 0.01 -0.02 0.01 -0.02 -0.47 -0.34 -0.24 -1.00
bv:topoW bv:topoE
nfN1
nfN2
nfN3
nfN4
nfN5
bv
topoW
topoE
topoLO
bv:topoW
bv:topoE 0.34
bv:topoLO 0.33 0.23
If we exclude the interaction, which would clearly be correlated with the single covariates, the rest of the coefficients are not much correlated. From this we may conclude that our assumption of independence holds true for this dataset.
We can also graphically check the independence of the error by simply plotting the residuals agains the fitted values and then fit a line through the points:
qplot(fitted(mod5), residuals(mod5), geom="point", xlab="Fitted Values", ylab="Residuals") +
geom_smooth(method = "lm", se = TRUE)
The result is the image below, which is simply another way to obtain the same result we saw for the ANCOVA but this time in ggplot2:
As you can see the line is horizontal, which means the residuals have no trend. Moreover, their spread is more or less constant for the whole range of fitted values. As you remember we assume the error term of the linear model to have zero mean and constant variance. For both these reason I think we can consider that the model meets both assumptions. However, if we color the points based on the variable topo (which is not shown but it is very easy to do with the option col in qplot) we can see that the 3-4 smaller clouds we see in the plot above are produced by particular topographical categories. This coupled with the fact that our data are probably autocorrelated, since they are sampled in space, may let us conclude that we should not assume independence and therefore GLS would be the best method.
In cases where the assumption of independence is violated, we would need to use a more robust method of maximum likelihood (ML) and residuals maximum likelihood (REML) for computing the coefficients. This can be done with the function
gls in the package
nlme, using the same syntax as for
lm:
mod6 = gls(yield ~ nf + bv * topo, data=dat, method="REML")
Anova(mod6, type=c("III"))
summary(mod6)
As you can see despite the different function (gls instead of lm), the rest of the syntax is the same as before. We can still use the function Anova to print the ANOVA table and summary to check the individual coefficients.
Moreover, we can also use the function anovato compare the two models (the one from glsand lm) and see which is the best performer:
> anova(mod6, mod5)
Model df AIC BIC logLik
mod6 1 14 27651.21 27737.18 -13811.61
mod5 2 14 27651.21 27737.18 -13811.61
The indexes AIC, BIC and logLik are all used to check the accuracy of the model and should be as low as possible. For more info please look at the appendix about assessing the accuracy of our model.
References and Further Reading
Finch, W.H., Bolin, J.E. and Kelley, K., 2014.
Multilevel modeling using R. Crc Press.
Yan, X. and Su, X., 2009.
Linear regression analysis: theory and computing. World Scientific.
James, G., Witten, D., Hastie, T. and Tibshirani, R., 2013.
An introduction to statistical learning (Vol. 6). New York: springer. http://www-bcf.usc.edu/~gareth/ISL/ISLR%20Sixth%20Printing.pdf
Long, J. Scott. 1997.
Regression Models for Categorical and Limited Dependent Variables. Sage. pp104-106. [For pseudo R-Squared equations, page available on google books]
Webster, R. and Oliver, M.A., 2007.
Geostatistics for environmental scientists. John Wiley & Sons.
West, B.T., Galecki, A.T. and Welch, K.B., 2014.
Linear mixed models. CRC Press.
Gałecki, A. and Burzykowski, T., 2013.
Linear mixed-effects models using R: A step-by-step approach. Springer Science & Business Media.
Williams, R., 2004.
One-Way Analysis of Variance. URL: https://www3.nd.edu/~rwilliam/stats1/x52.pdf
Witte, R. and Witte, J. 2009.
Statistics. 9th ed. Wiley.